
What is Nuclear Medicine ?
Nuclear Medicine is a medical specialty that uses small amounts of radioactive tracers – called radiopharmaceuticals- to create images of organs and lesions helping to treat diseases such as various cancers, cardiovascular and neurological disorders.
The imaging technics used in nuclear medicine work by injecting into the patient’s body targeted radiopharmaceuticals that accumulate within organs or lesions to reveal specific biochemical processes which help visualize how far the disease has spread or to see how well the treatment is working.

As the incidence of diseases such as cancer has increased worldwide so has the use of Nuclear Medicine facilitated by advancements in imaging equipment (SPECT and PET ) which has become a mandatory part of the protocols for the treatment of cancer patients.
Yet such techniques are still fundamentally dependent on the availability of the necessary radiopharmaceuticals. The production of such radiopharmaceuticals has proven to be complex and costly up to now.
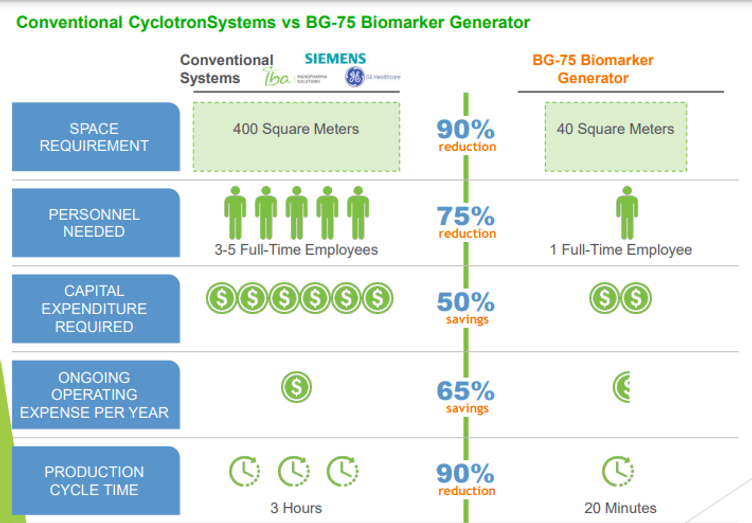
The minicyclotron BG75 fully automatic technology, offers the possibility to produce radiopharmaceuticals, “on demand”, safely, well established and economically within the hospital in a < 60m2 surface
ABT MOLECULAR IMAGING

BG-75"DOSE ON DEMAND" BIOMARKER GENERATOR
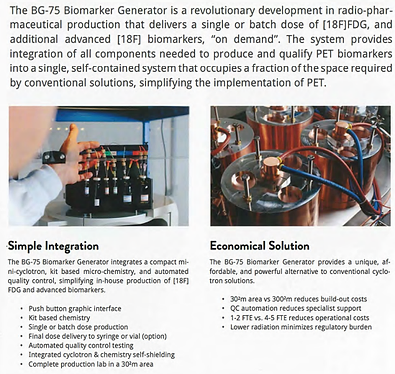

Fully Integrated Design
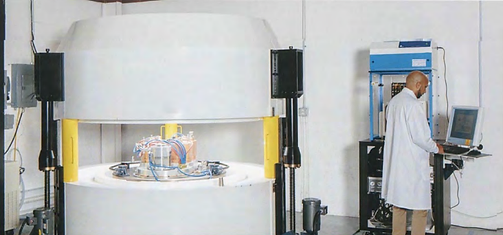
The BG-75 Biomarker Generator system integrates a 7.5 MeV cyclotron, Chemistry Production Module (CPM), and Quality Contrai Module (QCM) for on-site production of
[18F]FDG, providing automated production and quality contrai testing. Bath the cyclotron and chemistry mod ules are self-shielded, reducing radiation to <1 mR/hr at the minimum 5.5m x 5.5m room boundary.
Due to the system’s small footprint and self-shielding, the BG-75 Biomarker Generator can be easily incorporat ed into an existing clinical or research setting, adjacent to PET imaging equipment if needed. By contrast, stand ard PET biomarker laboratories produce batches of pos itron-emitting isotopes in a conventional medical cyclo tron, which poses a far greater radiation burden requiring significant physical containment of bath the cyclotron and ail downstream processing steps. Typically, a concrete-re-inforced bunker is specially built to contain the cyclotron, with separate « hot » labs dedicated to radiochemistry and QC, and several highly specialized staff to operate the cy clotron and perform the complex·functions. In compar ison, the BG-75 Biomarker Generator is scaled for a sin gle engineer/operator, occupies one-tenth the space, requires little infrastructure modification, and has embed ded chemistry and QC processes that greatly simplify the entire radiopharmaceutical production cycle.
These features translate into significantly Jess capital in vestment initially, and lower ongoing operating costs com pared to conventional PET biomarker laboratories. Addi tionally, due to its self-contained design and lower energy, decommissioning the system at the end of its useful life is much simpler and far less costly. Overall, the total cost of ownership for the BG-75 Biomarker Generator is less than one fourth that of conventional cyclotron solutions.

Automated Production
The BG-75 Chemistry Module greatly simplifies the workflow associated with radiopharmaceutical production by miniaturizing and automating the processes for biomarker radiolabeling and quality control. The system is provided with necessary consumables for daily operation including Dose Synthesis Cards and Reagent Kits for the biomarker synthesis, and maintenance, cleaning, and SST cards for quality control calibration.
The [18F] FDG production kit contains two different size daily. Reagents Kits to meet your site’s needs, and support scability. The standard Chemistry Module supports clinical [18F]FDG dose production.
With the NDB synthesizer, you can be the Best with 15 different tracers available : [18F]FDG, [18F]FMiso, [18F]FLT, [18F]FCholine, [18F]FAcetate, [18F]FET, [18F]FES, [18F]FDGal, [18F]FDopa, [18F]PSMA-1007, [18F]SFB, [18F]MPPF and [18F]FHBG…
True to its vision of expanding the use and usefulness of PET around the globe, OCELO HEALTH CARE seeks to fully support both prospective and committed customers throughout the entire lifecycle of the client relationship. Recognizing that many BG 75 customers may be new to PET, and the unique logistical as well as regulatory considerations in evaluating and implementing technology that involves radioactive drugs, OCELO HEALTH CARE offers a comprehensive suite of services that enables customers to maximize value from their investment.
HiPro
BG-75 Biomaker Generator Option

Faster, easie and more cost effective FDG Production
BG 75 ‘s Advantages
⦁ Point of Care :FDG dose available to inject in patient within 1 hour, not dispensed hours after production with conventional cyclotron bulk production
⦁ Requires much less space than conventional cyclotron solutions, and requires no quality control lab.
⦁ Only system to integrate automated QC, while conventional solutions require a full QC Lab and the radiochemist expertise to conduct testing.
⦁ No additional shielding required. Even self-shielded conventional cyclotrons require additional concrete due to high radiation bulk production vs. the BG-75 “on-demand” production.
⦁ Lower repair costs and less breakage / down time due to lower radiation, internal targets, and on-demand vs. bulk production
⦁ Less operating cost, and does not require advanced technical personnel to operate
⦁ Point of Care :FDG dose available to inject in patient within 1 hour, not dispensed hours after production with conventional cyclotron bulk production
⦁ Requires much less space than conventional cyclotron solutions, and requires no quality control lab.
⦁ Only system to integrate automated QC, while conventional solutions require a full QC Lab and the radiochemist expertise to conduct testing.
⦁ No additional shielding required. Even self-shielded conventional cyclotrons require additional concrete due to high radiation bulk production vs. the BG-75 “on-demand” production.
⦁ Lower repair costs and less breakage / down time due to lower radiation, internal targets, and on-demand vs. bulk production
⦁ Less operating cost, and does not require advanced technical personnel to operate
Integrated Solution: "Dose-on-Demand" BG-75 Biomaker Generator

⦁ The BG-75 Biomarker Generator integrates a radioisotope generator with kit-based micro-radiochemistry and automated quality control to provide PET biomarkers at a user’s fingertip
Simple graphic user interface navigates the user through the production process while embedded production and quality control processes minimize the need for specialized staff
⦁ The BG-75’s self-shielding, small size and low power requirements allow for a simple installation with minimal facility modifications
A complete PET biomarker lab can fit within a 35 m2 space
Self-shielded accelerator and chemistry produces a low radiation burden
Minimal modifications to a facility are needed to implement, resulting in a quick and low cost installation
⦁ The BG-75 is scaled for a single user and is a cost effective solution to either introduce or expand the use of PET within a facility
Consumable reagent kits and dose synthesis cards make it well suited for dose-on-demand
Low infrastructure requirements dramatically reduce operating costs
⦁ The BG-75 produces the critical PET biomarker, FDG, for today’s clinical needs and is easily adaptable for future radioisotopes and PET biomarkers of tomorrow
Single and dual-dose production of an FDG dose as fast as every 20 minutes or small batch production every hour.
Compact 7.5 MeV Mini-Cyclotron
Advanced F-18 biomarker capability including FMISO, FLTand more….

BG-75 Micro-Chemistry Unit
⦁ Key Features
Final Radioactivity Yield: 10-13 mCi’s single dose, 26-28 mCi’s batch dose
Final Product Volume: 2.0 ml
Control System: HMI with embedded control
Consumables:
⦁ Reagent kits, containing chemicals for radiolabeling reaction
⦁ Sterile, disposable, single-use Dose Synthesis Cards containing production components
Compact 7.5 MeV Mini-Cyclotron
Programmable and capable of producing any one- or two-step F-18 radiochemistry process
Self-shielded, installed adjacent to the accelerator shield
⦁ Quality Control
Embedded quality control process minimizes the need for highly specialized staff
System uses embedded methods, micro-sensors, and small-scale analytics (HPLC, radiation detector, pH meter) to perform multiple tests as required by pharmacopeia standards to qualify radiopharmaceutical for human injection
⦁ pH
⦁ Filter integrity
⦁ Residual volatile organics
⦁ Radiochemical purity
⦁ Radiochemical identity
⦁ Chemical Purity

BG-75 Shielding
⦁ Key Features
Material Casing: ¼” steel
Shielding Material: Dense concrete and boronated polyethylene
Diameter: 2.39 m
Height: 1.63 m
Weight: 21 tons
Radiation Field Room Boundary: <1 mR/hour
Self-shielding for generator and chemistry minimizes exposure, eliminates the need for additional lead enclosures, and reduces facility modifications
Opens vertically for servicing

BG-75 Mini-Cyclotron
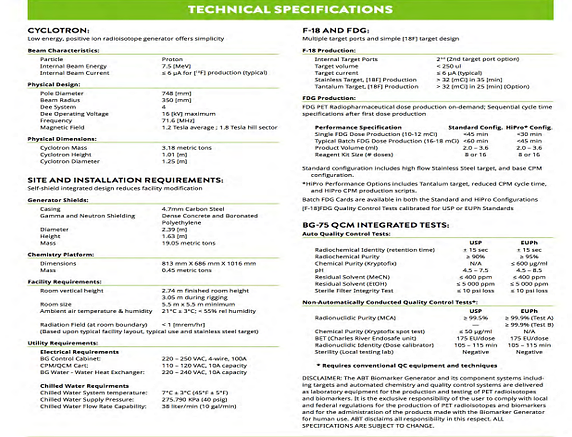
⦁ Beam Characteristics Particle: Proton
Paticle : Proton
Internal Beam Energy: 7.5 MeV
Internal Beam Current: <5 uA for F-18 production
Internal Target Ports: 3 (non-simultaneous)
⦁ Physical Design
Pole Diameter: 74.8 mm
Extraction Radius: 35 cm
Dee System: 4
Dee Operating Voltage: 16 kV max
Frequency: 72 MHz
Magnetic Field: 1.2 Tesla avg. / 1.8 Tesla max
⦁ Physical Dimensions
Magnet Mass: 3.5 tons
Cyclotron Height: 0.37 m
Cyclotron Diameter: 1.25 m
⦁ Other Key Features
Low power
Simple one button operation and fast production Access to target
and ion source for simple
replacement
Turbo pump versus oil-based diffusion pump
Lower regulatory burden due to less radiation exposure
« The GLOBOCAN 2020 database, accessible online as part the IARC Global Cancer Observatory, provides estimates for 2020 of incidence and mortality in 185 countries for 36 types of cancer and for all cancer sites combined.
The global cancer burden is estimated to have risen to 19.3 million new cases and 10.0 million deaths in 2020.
One in 5 people worlwide develop cancer during their lifetime, and one in 8 men and one in 11 women die from disease. »
Source International Agency for Research on Cancer – 15 December 2020
« The GLOBOCAN 2020 database, accessible online as part the IARC Global Cancer Observatory, provides estimates for 2020 of incidence and mortality in 185 countries for 36 types of cancer and for all cancer sites combined.
The global cancer burden is estimated to have risen to 19.3 million new cases and 10.0 million deaths in 2020.
One in 5 people worlwide develop cancer during their lifetime, and one in 8 men and one in 11 women die from disease. »
Source International Agency for Research on Cancer – 15 December 2020
Conventional CyclotronSystems vs BG-75 Biomaker Generator


OUR SCIENTIFIC COMMITEE
OUR SCIENTIFIC COMMITEE
Benelux.EU HEALTH CARE and PHARMA NUCLEAR SERVICES partnership and its Scientific Committee will assist in the establishment and the proper functioning of the new PET Center in your country.
The use of Nuclear Medicine, facilitated by the significant advancements of imaging equipment like PET, has become a mandatory and a crucial part of diagnostic, treatment protocols and the evaluation of their efficacy. These therapeutic decisions imply the highest quality of the examinations.
Participation in clinical trials will be possible if the PET center complies with international standards.
The scientific committee consists of 7 experts, led by Professor Denis Guilloteau ( Head of in vitro Nuclear Medicine Department and Director of Inserm U1253 “Imaging and Brain “ ( iBrain) of Tours University Hospital Bretonneau (France) will bring their support to the following phases:
⦁ PET center set-up: installation, compliance with radioprotection regulations and pharmaceuticals standards; equipment and human resources.
⦁ Training in the production of radiopharmaceuticals (synthesis, control, dispensing) Although BEST-ABT system is automated, the PET center personnel must be familiar with the fundamental principles such as isotopes production in a cyclotron, the synthesis, the specific biomarkers, the quality assurance and quality control system amongst others.
⦁ Conduct of medical examinations in line with protocols
⦁ Interpretation of images
⦁ Relationships with requesting departments and the organisation of internal meetings
Benelux.EU HEALTH CARE and PHARMA NUCLEAR SERVICES partnership and its Scientific Committee will assist in the establishment and the proper functioning of the new PET Center in your country.
The use of Nuclear Medicine, facilitated by the significant advancements of imaging equipment like PET, has become a mandatory and a crucial part of diagnostic, treatment protocols and the evaluation of their efficacy. These therapeutic decisions imply the highest quality of the examinations.
Participation in clinical trials will be possible if the PET center complies with international standards.
The scientific committee consists of 7 experts, led by Professor Denis Guilloteau ( Head of in vitro Nuclear Medicine Department and Director of Inserm U1253 “Imaging and Brain “ ( iBrain) of Tours University Hospital Bretonneau (France) will bring their support to the following phases:
⦁ PET center set-up: installation, compliance with radioprotection regulations and pharmaceuticals standards; equipment and human resources.
⦁ Training in the production of radiopharmaceuticals (synthesis, control, dispensing) Although BEST-ABT system is automated, the PET center personnel must be familiar with the fundamental principles such as isotopes production in a cyclotron, the synthesis, the specific biomarkers, the quality assurance and quality control system amongst others.
⦁ Conduct of medical examinations in line with protocols
⦁ Interpretation of images
⦁ Relationships with requesting departments and the organisation of internal meetings

THE MAINTENANCE
THE MAINTENANCE
You access a new generation of customized services bundle
⦁ Specially designed to attend your needs
⦁ Managed through our own network
A MAINTENANCE TECHNICIAN ON SITE
We will build close relationships and help you to achieve your performance objectives:
⦁ On-site dedicated maintenance technician
⦁ Quick remote access to our OCELO/PNS experts
⦁ Fast responses associated with an on-site intervention plan
⦁ Proactive maintenance and getting an optimal performance level
⦁ Enjoying privileged assistance and guarantied upgrades
⦁ Online clinical training
A TOTAL AVAILABILITY TO HELP MANAGE YOUR EQUIPMENT
The objective is to maximize your productivity and return on your investment through:
⦁ Optimal warranty for you equipment
⦁ Fast responses associated with an on-site intervention plan
⦁ Proactive monitoring and maintenance program
⦁ Priority services
FULL COVERAGE
We respect you budget with high quality assistance and cost-effectiveness with:
⦁ A reliable assistance and short response time
⦁ A guarantied on-site fast intervention time
⦁ A timely intervention planning notification
⦁ An privileged access to OCELO/PNS experts
You access a new generation of customized services bundle
⦁ Specially designed to attend your needs
⦁ Managed through our own network
A MAINTENANCE TECHNICIAN ON SITE
We will build close relationships and help you to achieve your performance objectives:
⦁ On-site dedicated maintenance technician
⦁ Quick remote access to our OCELO/PNS experts
⦁ Fast responses associated with an on-site intervention plan
⦁ Proactive maintenance and getting an optimal performance level
⦁ Enjoying privileged assistance and guarantied upgrades
⦁ Online clinical training
A TOTAL AVAILABILITY TO HELP MANAGE YOUR EQUIPMENT
The objective is to maximize your productivity and return on your investment through:
⦁ Optimal warranty for you equipment
⦁ Fast responses associated with an on-site intervention plan
⦁ Proactive monitoring and maintenance program
⦁ Priority services
FULL COVERAGE
We respect you budget with high quality assistance and cost-effectiveness with:
⦁ A reliable assistance and short response time
⦁ A guarantied on-site fast intervention time
⦁ A timely intervention planning notification
⦁ An privileged access to OCELO/PNS experts
THE PACK EQUIPMENT

THE PACK EQUIPMENT
We offer a “blue print” project for a complete Nuclear Medicine facilities project in order to validate the technical installation and identify the team of technicians who will be involved in the department. Our team will bring the required support and provide specific training programs.
The Pack Equipment includes:
⦁ The PET/SCAN: an absolute requirement
⦁ The Mini cyclotron: the essential complement to the PET/SCAN
⦁ Additional Laboratory Equipment for routine operation and maintenance of the BG-75 System and PET/SCAN
⦁ Additional Planning Equipment for PET/SCAN and BG-75 System
Space required:
⦁ For the diagnostic part: +- 100 m² for the PET/SCAN and 60 m² the mini cyclotron
⦁ For the radiotherapy part (in option): 204 m² ( in a bunker) The PET center will be the center of excellence for a
national and international visibility offering to patients the best diagnostic and therapeutic protocols.
We offer a “blue print” project for a complete Nuclear Medicine facilities project in order to validate the technical installation and identify the team of technicians who will be involved in the department. Our team will bring the required support and provide specific training programs.
The Pack Equipment includes:
⦁ The PET/SCAN: an absolute requirement
⦁ The Mini cyclotron: the essential complement to the PET/SCAN
⦁ Additional Laboratory Equipment for routine operation and maintenance of the BG-75 System and PET/SCAN
⦁ Additional Planning Equipment for PET/SCAN and BG-75 System
Space required:
⦁ For the diagnostic part: +- 100 m² for the PET/SCAN and 60 m² the mini cyclotron
⦁ For the radiotherapy part (in option): 204 m² ( in a bunker) The PET center will be the center of excellence for a
national and international visibility offering to patients the best diagnostic and therapeutic protocols.

CONTACTS
CONTACTS
Benelux.EU HealthTech Innovations
WEBSITE : www.ocelohealthcare.com
WEBSITE : www.healthtech-innovations.be
M/s. BENELUX BD. INTERNATIONAL – Ahmed NUR – Managing Partner
DHAKA OFFICE : H-54/24, R-12, P.C. CULTURE HOUSING
BELGIUM OFFICE : Avenue Jules de Trooz 61, 1150, Woluwe Saint-Pierre Belgium
Benelux.EU HealthTech Innovations
WEBSITE : www.ocelohealthcare.com
WEBSITE : www.healthtech-innovations.be
M/s. BENELUX BD. INTERNATIONAL – Ahmed NUR – Managing Partner
DHAKA OFFICE : H-54/24, R-12, P.C. CULTURE HOUSING
BELGIUM OFFICE : Avenue Jules de Trooz 61, 1150, Woluwe Saint-Pierre Belgium
